Medical Devices Vigilance Market: Ensuring Safety and Compliance in Healthcare Devices
Medical Devices Vigilance Market: Ensuring Safety and Compliance in Healthcare Devices
Blog Article
"Medical Devices Vigilance Market Size And Forecast by 2031
Graphs and data visuals in the research Medical Devices Vigilance Market report provide a detailed understanding of market size, demand, and revenue patterns. These insights help companies develop strategies to capture a larger market share. Industry statistics reveal the growing importance of innovation and sustainability in shaping industry trends. Leaders in the market are focusing on enhancing their offerings to align with these trends and meet consumer expectations effectively.
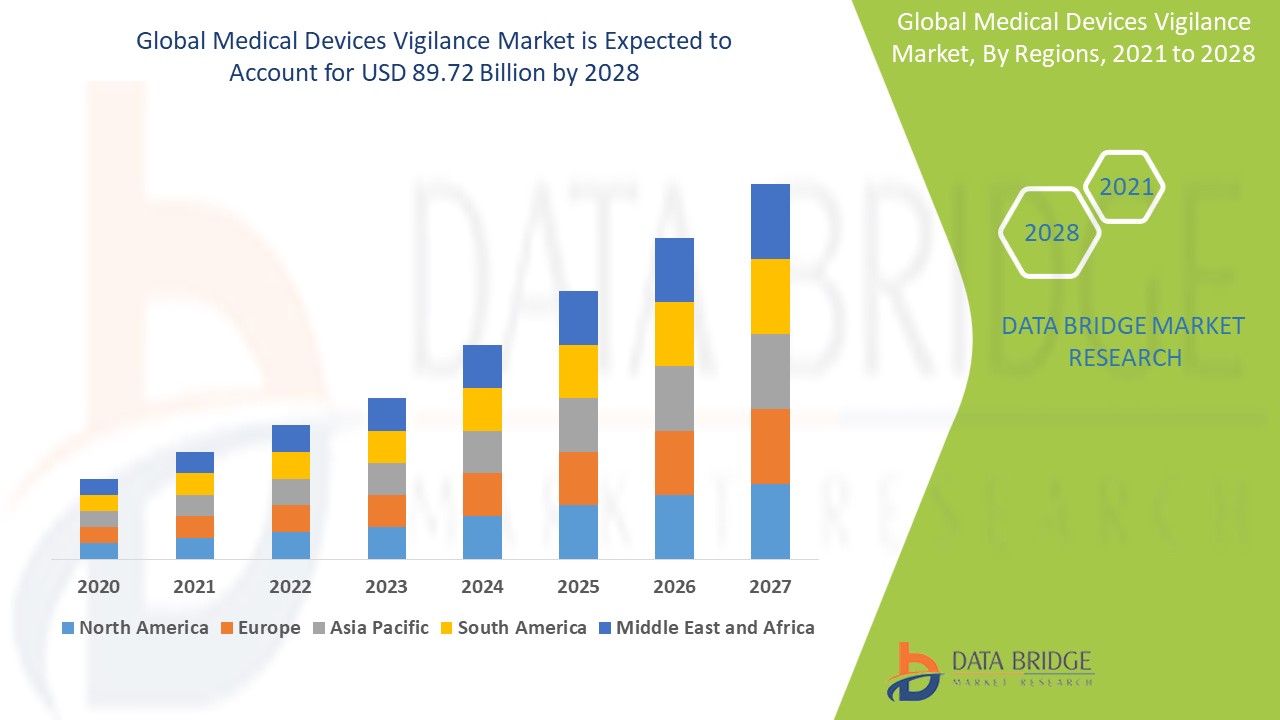
Medical devices vigilance market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses the market to account to USD 89.72 billion by 2028 growing at a CAGR of 6.89% in the above-mentioned forecast period.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-devices-vigilance-market
Which are the top companies operating in the Medical Devices Vigilance Market?
The global Medical Devices Vigilance Market study presents a detailed analysis of the industry, focusing on key trends, market dynamics, and the competitive landscape. It highlights leading companies in the market, examining their strategies and contributions to market share. Additionally, the report offers insights into the Top 10 Companies in Medical Devices Vigilance Market in the Medical Devices Vigilance Market, including their business strategies, financial performance, and overall market position.
**Segments**
- Based on type, the global medical devices vigilance market can be segmented into software and services. The software segment is anticipated to witness significant growth due to the increasing demand for vigilance software solutions that can effectively monitor and report adverse events related to medical devices. On the other hand, the services segment is also expected to grow as more healthcare facilities are outsourcing vigilance activities to specialized service providers to ensure compliance with regulatory requirements.
- By application, the market can be categorized into hospitals, clinics, ambulatory surgical centers, and others. Hospitals are expected to dominate the market as they are the primary users of medical devices and are responsible for monitoring the safety and performance of these devices. Clinics and ambulatory surgical centers are also significant end-users of medical devices vigilance solutions, driving the demand for advanced software and services in these settings.
- Geographically, the global medical devices vigilance market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America is expected to hold a substantial market share due to stringent regulatory requirements, increasing incidents of adverse events, and the presence of key market players in the region. Europe is also a major market for medical devices vigilance, driven by the growing emphasis on patient safety and the adoption of advanced healthcare technologies.
**Market Players**
- Some of the key players in the global medical devices vigilance market include IBM Corporation, Sparta Systems, AssurX, Oracle, Sarjen Systems Pvt. Ltd., ZEINCRO, Online Business Applications, Inc., EXTEDO GmbH, and Saratech Consultants & Engineers. These companies are focusing on strategic partnerships, product innovations, and geographic expansion to strengthen their market presence and cater to the evolving needs of healthcare providers and regulators.
- Other notable market players include Axendia, Factorytalk Co., Ltd., iConcept, Jouve-Gens, Pixel Medical Solutions, PRIMARA Test- und Zertifizier-GmbH, QMSIn the global medical devices vigilance market, key players such as IBM Corporation, Sparta Systems, AssurX, Oracle, and Sarjen Systems Pvt. Ltd. are at the forefront of driving innovation and advancing healthcare quality through their vigilance solutions. These companies have established themselves as leaders in the industry by offering cutting-edge software and services that enable healthcare facilities to monitor and report adverse events related to medical devices effectively. Through strategic partnerships and product innovations, these market players are continuously striving to enhance their offerings and meet the evolving needs of healthcare providers and regulators.
Additionally, companies like ZEINCRO, Online Business Applications, Inc., EXTEDO GmbH, and Saratech Consultants & Engineers are also significant players in the medical devices vigilance market, contributing to the overall growth and development of the industry. These companies bring a unique set of capabilities and expertise to the market, offering a diverse range of solutions tailored to the specific requirements of healthcare organizations. By expanding their geographic footprint and focusing on customer-centric approaches, these market players are positioning themselves for sustained success in the competitive landscape of medical devices vigilance.
Furthermore, emerging players in the market such as Axendia, Factorytalk Co., Ltd., iConcept, Jouve-Gens, Pixel Medical Solutions, PRIMARA Test- und Zertifizier-GmbH, and QMS are also making significant strides towards driving innovation and disruption in the medical devices vigilance space. These companies are leveraging technology advancements and industry best practices to introduce novel solutions that address the growing complexity of vigilance requirements in healthcare settings. By offering new insights and perspectives on vigilance processes and compliance management, these emerging market players are reshaping the future of medical devices vigilance and setting new benchmarks for quality and safety standards.
Overall, the global medical devices vigilance market is witnessing a rapid transformation driven by technological advancements, regulatory compliance mandates, and the increasing focus on patient safety. Market players across all segments are continuously innovating and collaborating to address the dynamic needs**Market Players**
The major players covered in the medical devices vigilance market report are AB-Cube, AssurX, Inc., Oracle, Sarjen Systems Pvt. Ltd., Sparta Systems, Inc., Xybion Corporation, ZEINCRO Group, Arena Solutions, Inc., Intel Corporation, mdiConsultants, Inc., EXTEDO, Freyr, Greenlight Guru, Jama Software, RELX Group plc, MasterControl, Inc., Panacea Pharma Projects Limited, Smithers, Laerdal Medical, Qvigilance, among other domestic and global players. These market players are actively involved in driving innovation and advancing healthcare quality through their vigilance solutions. Market share data is available for Global, North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South America separately. The competitive analysis provided for each competitor individually by DBMR analysts helps in understanding the competitive strengths and strategic positioning of key players in the market.
The global medical devices vigilance market is witnessing a rapid transformation fueled by technological advancements, regulatory compliance mandates, and the increasing emphasis on patient safety. Market players across all segments are continuously innovating and collaborating to address the dynamic needs of healthcare providers and regulators. Companies like IBM Corporation, Sparta Systems, AssurX, Oracle, and Sarjen Systems Pvt. Ltd. are leading the market by offering cutting-edge software and services to effectively monitor and report adverse events related to medical devices. Through strategic partnerships and product innovations, these
Explore Further Details about This Research Medical Devices Vigilance Market Report https://www.databridgemarketresearch.com/reports/global-medical-devices-vigilance-market
Why B2B Companies Worldwide Choose Us for Revenue Growth and Sustainability
- Gain a clear understanding of the Medical Devices Vigilance Market, its operations, and stages in the value chain.
- Explore the current market scenario and assess future growth potential throughout the forecast period.
- Strategize effectively for marketing, market entry, expansion, and business plans by analyzing growth factors and buyer behavior.
- Stay ahead of competitors by studying their business models, strategies, and prospects.
- Make data-driven decisions with access to comprehensive primary and secondary research.
Key Insights from the Global Global Medical Devices Vigilance Market :
- Comprehensive Market Overview: A detailed examination of the global Medical Devices Vigilance Market.
- Industry Trends and Projections: Analysis of historical data (2015 onward) and future growth forecasts, including compound annual growth rates (CAGRs).
- Emerging Opportunities: Identification of new market prospects and targeted marketing strategies.
- Focus on R&D: Insights into demand for new product launches and innovative applications.
- Leading Player Profiles: Detailed profiles of major market participants.
- Market Composition: Analysis of dynamic molecule types, targets, and key resources.
- Revenue Growth: Examination of global market revenue, segmented by key players and product categories.
- Commercial Opportunities: Analysis of sales trends, licensing deals, and co-development opportunities.
Regional Insights and Language Accessibility
- North America: United States, copyright, Mexico
- Europe: Germany, France, UK, Russia, Italy
- Asia-Pacific: China, Japan, Korea, India, Southeast Asia
- South America: Brazil, Argentina, Colombia, and others
- Middle East and Africa: Saudi Arabia, UAE, Egypt, Nigeria, South Africa
Understanding market trends at a regional level is crucial for effective decision-making. Our reports cater to diverse audiences by offering localized analyses in multiple regional languages. These reports provide tailored insights for specific regions, enabling businesses and stakeholders to access relevant information for informed strategies. By bridging communication gaps, we empower regional markets to thrive and grow. Access our reports in your preferred language for a personalized understanding of industry dynamics.
Japanese : https://www.databridgemarketresearch.com/jp/reports/global-medical-devices-vigilance-market
Chinese : https://www.databridgemarketresearch.com/zh/reports/global-medical-devices-vigilance-market
Arabic : https://www.databridgemarketresearch.com/ar/reports/global-medical-devices-vigilance-market
Portuguese : https://www.databridgemarketresearch.com/pt/reports/global-medical-devices-vigilance-market
German : https://www.databridgemarketresearch.com/de/reports/global-medical-devices-vigilance-market
French : https://www.databridgemarketresearch.com/fr/reports/global-medical-devices-vigilance-market
Spanish : https://www.databridgemarketresearch.com/es/reports/global-medical-devices-vigilance-market
Korean : https://www.databridgemarketresearch.com/ko/reports/global-medical-devices-vigilance-market
Russian : https://www.databridgemarketresearch.com/ru/reports/global-medical-devices-vigilance-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975
Email:- corporatesales@databridgemarketresearch.com" Report this page